AI Rocketfuel for Your Document Management.
Fieldwise provides developers with a fast and intuitive platform to modernize and streamline complex document workflows.
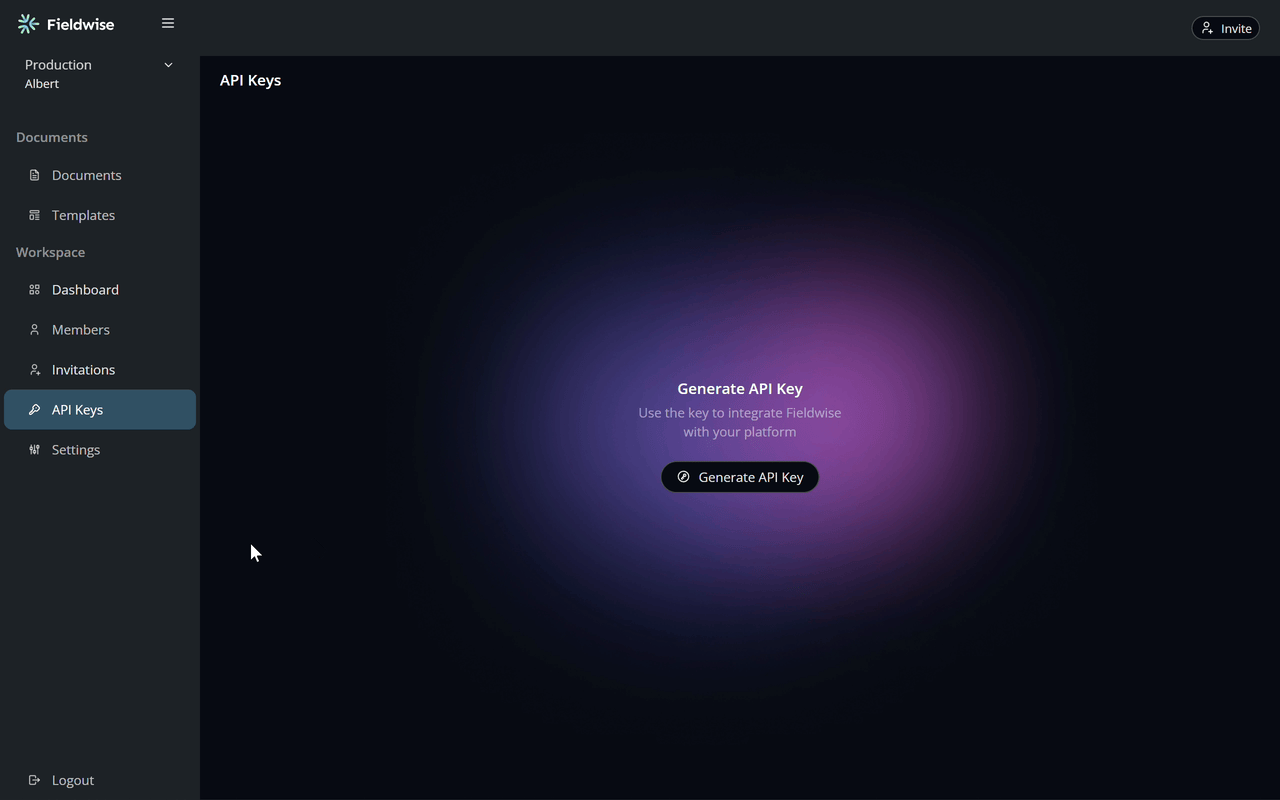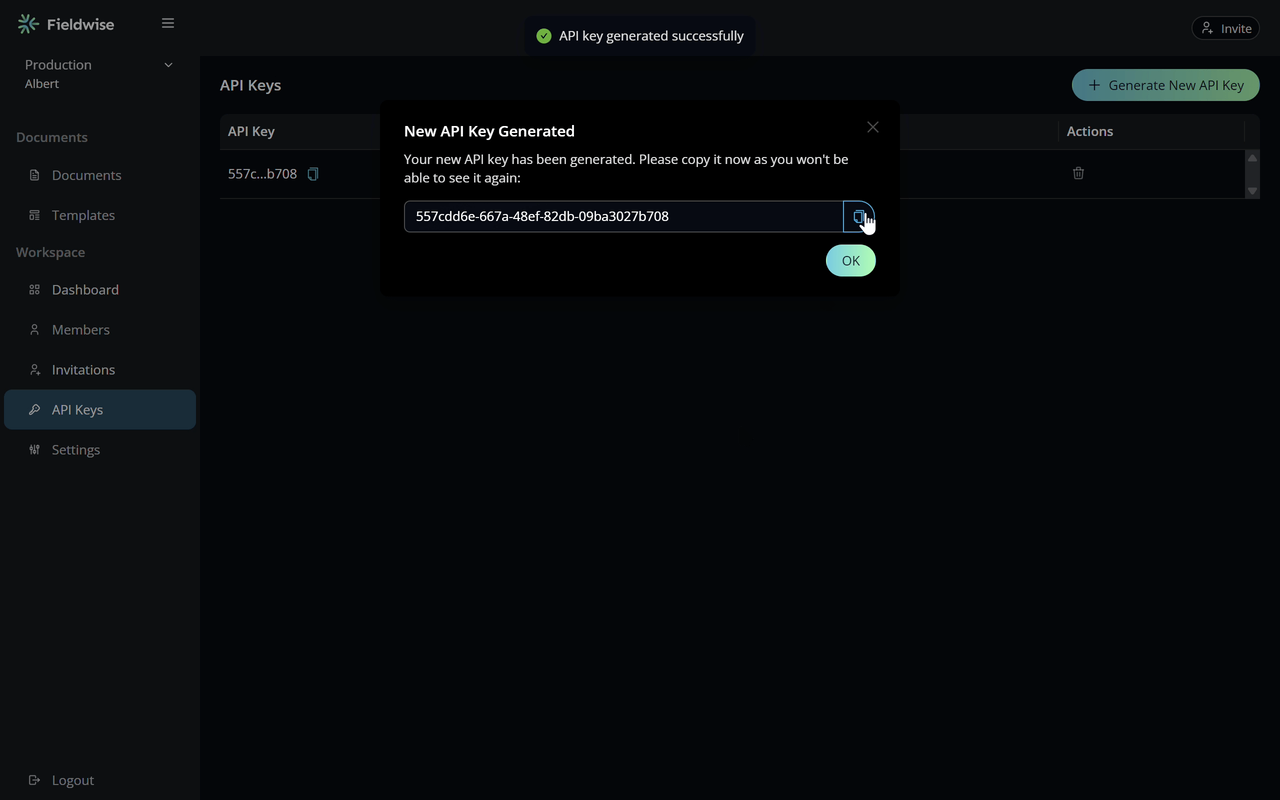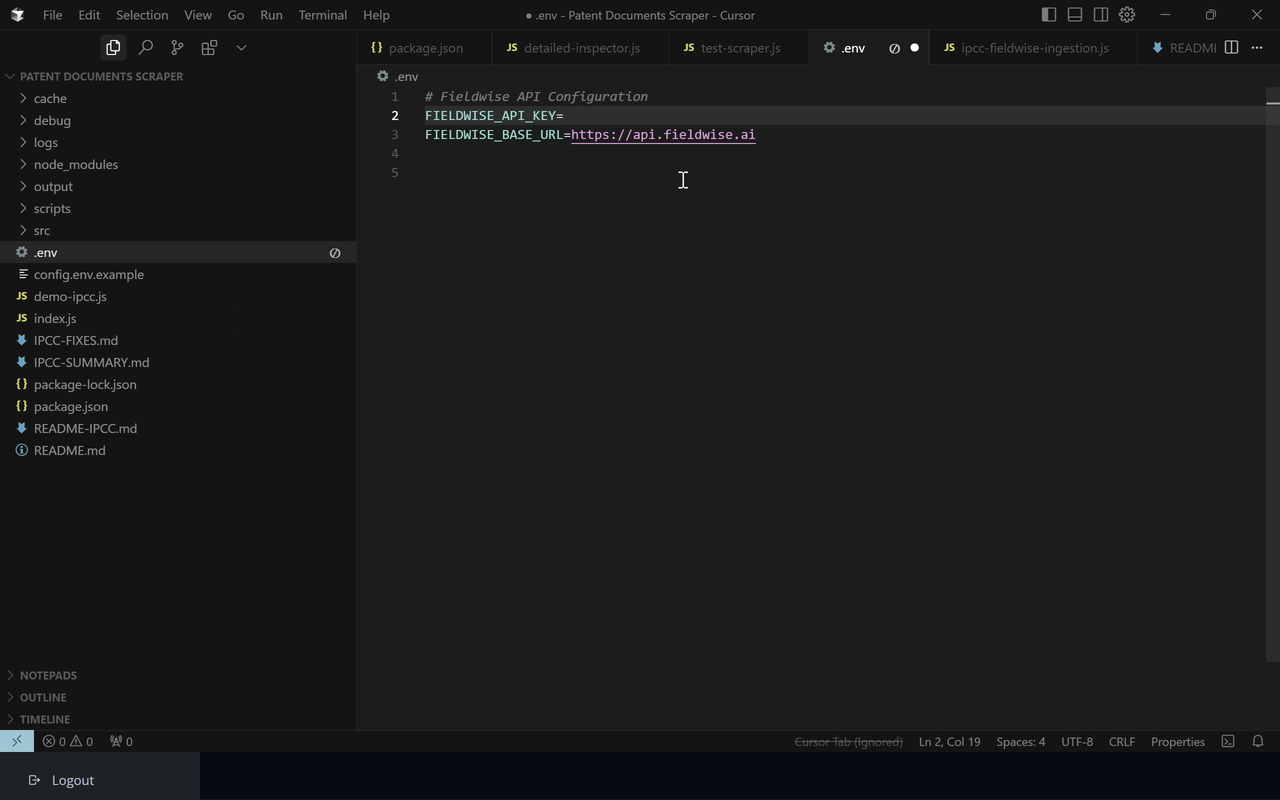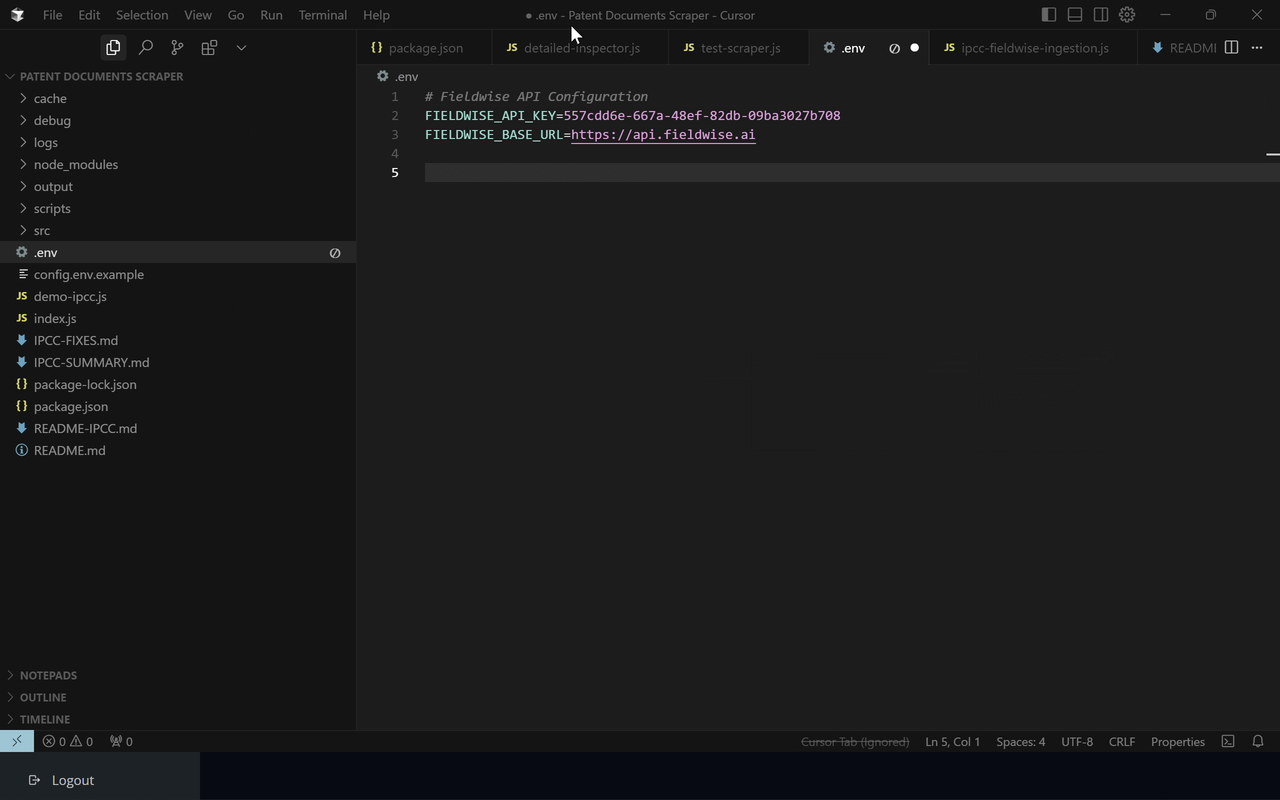
Plug in your API key to start processing data seamlessly with Fieldwise
Add your API key and configure settings right in your project—Fieldwise takes care of syncing and managing your data securely and efficiently.
By Developers For Developers
Fieldwise is a modern document intelligence platform designed to integrate with new and existing document management workflows and systems. We built Fieldwise for developers. As such, we offer:
Fast Deployment
Accessible Documentation
Automatic Syncing
Your data is safe and sound with our end-to-end encrypted security system
Our data is safely stored in AWS. We ensure that our databases (RDS) and files (S3) are safeguarded with encryption both at rest and during transmission, along with stringent access controls and routine backups.